Longhorn Vaccines & Diagnostics LLC
Longhorn Vaccines and Diagnostics, LLC is at the forefront of molecular testing, vaccine development and diagnostics solutions. With an emphasis on innovation, their mission is to improve molecular diagnostics for global public health concerns. Their unique diagnostic solutions thrive in a wide variety of environments, helping to diagnose and treat infectious diseases such as Influenza, SARS-CoV-2, Monkeypox, and Mycobacterium Tuberculosis (MTB).
View All Longhorn Vaccines & Diagnostics LLC Products
PrimeStore® MTM
Their patented, FDA cleared PrimeStore® MTM (Molecular Transport Media) has been a game changer, proven to completely inactivate viruses, fungal and bacterial pathogens including challenging, high consequence pathogens/foreign animal diseases in different sample matrices with high nucleic acid transport stability even at high-temperatures thereby providing high quality nucleic acids for RT-PCR and Sequencing including targeted NGS and whole genome sequencing. During the COVID-19 pandemic, PrimeStore® MTM played a pivotal role in sample collection, saving lives globally with weekly shipments having exceeded two million tubes.
PrimeStore® MTM
- Inactivate/kill infectious biological pathogens, including MTB, gram positive/negative bacteria, fungi, and viruses.
- Disrupt/lyse lipid membranes
- Destroy proteins and enzymes
- Inactivate nucleases and proteases
- Preserve/stabilise naked DNA
- Maintain high quality nucleic acid
- Stabilise released RNA/DNA for downstream molecular testing and sequencing
- Be compatible with most RNA/DNA isolation kits
- Shipment without dry ice or cold chain
- Stabilise RNA/DNA at elevated temperatures
- Unaffected by to freeze/thaws
Key Benefits of PS-MTM
Stability Study:
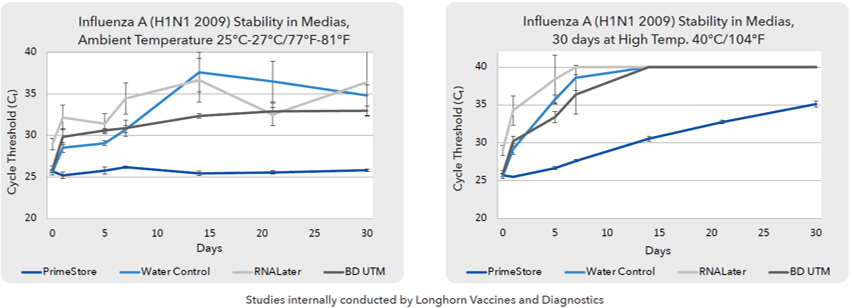
- Stability in Transportation even after 30-day duration in high temperature environments: Influenza A clinical samples collected in PS-MTM have proven to offer higher stability with optimum Ct value even at high temperature storage even after 30 days compared to other stored samples.
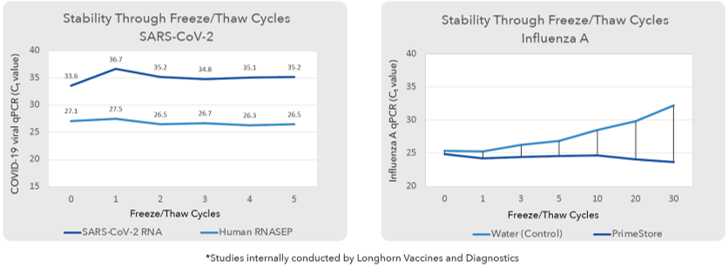
- Optimum Stability after multiple freeze thaw process
Stored SARS-CoV-2 clinical swabs in PS-MTM and Influenza A clinical swab collected in PS-MTM showed optimum Ct value compared to Control after multiple freeze thaw process keeping nucleic acids intact
Publications
Longhorn’s PS-MTM usage for inactivated sample transportation and storage has been successfully published in more than 100 publications and have also been compatible with the UK Government guidance in usage at NHS Pathology clinics in improving the process on Point of Care systems.
Customer Insights
Improving Process on POC Systems
| Regarding the use of inactivated samples on the cobas® Liat® by NHS Pathology Solutions, Validation Report, Dec 2020: |
The use of these samples (In VTM) would require all processing and pipetting to take place in a microbiological safety cabinet (MSC) at containment level 2 (CL2) or greater, to be compliant with government guidance. Alternatives for VTM need to be explored to permit deactivation of the live virus in samples prior to analysis, to allow analysis at true Point of Care settings.
| "A decentralized point-of-care testing model to address inequities in the COVID-19 response". From Australia in Lancet, Dec. 2020: |
During the programme, we changed from using a universal viral transport medium to a molecular transport medium (Longhorn, TX, USA), which preserves the integrity of the RNA, is useful for transport of specimens, an inactivates the virus, rendering the sample non-infectious, thereby increasing POC test operator safety.
Specimen Collection Procedure:
Step 1-2: Samples are collected by using a swab for all sample types. Depending on the sample type a certain collection technique needs to be incorporated when using swabs.
Step 3: Insert the flocked swab directly into the solution and break of excess swab handle.
Step 4: Close tightly. Place at RT until ready to ship to a diagnostic laboratory. MTB DNA from samples collected and stored in PS-MTM is stable for up to 30 days at ambient temperature (10-26oC). Influenza A RNA from samples collected and stored in PS-MTM is stable for up to 7 days at ambient temperature (26oC) or can be refrigerated for up to 28 days. Proceed with RNA/DNA extraction after letting the specimen sit in PS-MTM for a minimum of 60 minutes. Vortex sample before use. Extract the RNA/DNA using an extraction kit, or an automated platform validated for use with PS-MTM.
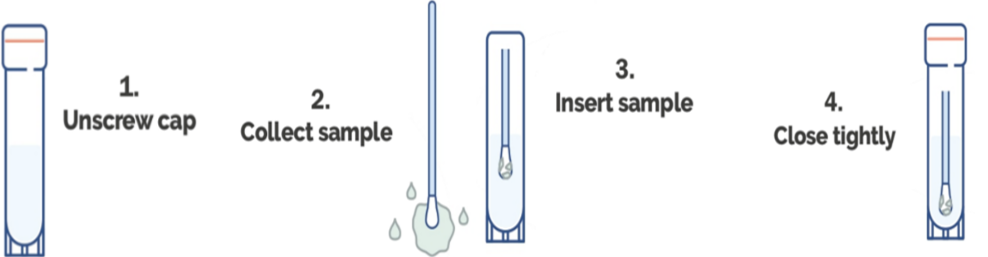
Product Use
Precautions when using PrimeStore MTM (PS-MTM) form Product Insert (IFU)
To be used by trained and qualified professionals. Federal Law restricts this device to sale by or on the order of a licensed medical professional (human or animal) by law in the state in which said professional practices, to use or order the use of this device.
- WARNING: Guanidine Thiocyanate forms very toxic gases on contact with acids! Keep away from acidic or alkaline products and oxidising agents.
- Do NOT use bleach for cleaning or disinfection. Not to be used with lab automation systems that use bleach including the Hologic Panther and Fusion systems.
- Do NOT breathe vapours.
- Avoid contact with eyes and skin.
- Do NOT insert swab into solution before collecting patient specimen.
- Do NOT drink, touch or remove PS-MTM from collection tube.
- Do NOT transfer PS-MTM into other tubes.
- Do NOT pool PS-MTM into larger volumes or leave tubes uncapped for more than 10 minutes.
- For specimens in PS-MTM follow state, local and institution guidelines for the handling and disposal of biohazardous waste.
Cleaning up & Disposing of PrimeStore MTM
Safety Guidelines
- In case of a spill from a tube of PrimeStore MTM, please follow your Laboratory/Institution Safety Guidelines for the handling and disposal of nucleic acid extraction kits that use guanidine. DO NOT USE BLEACH.
Cleanup Procedure
- The spill can immediately be cleaned up with a dry wipe or laboratory detergent and water, followed by an alcohol or ethanol wipe. Dispose of the wipes in the appropriate biohazard waste disposal bin. DO NOT USE BLEACH.
Disposal
- Longhorn recommends that Labs processing samples collected in PS-MTM handle all waste by following their own Waste Disposal Protocols for their routine nucleic acid extraction kits (for DNA or RNA) since one component in PrimeStore MTM aligns with the percentage of guanidine content in all Lysis Buffers.
When NOT TO USE PrimesStore® MTM
- You need to culture
- You need to do antigen/antibody testing
- You need to work with proteins
- You use a platform with a bleach step (i.e Hologic Panther)
- You use platforms that do not have a nucleic acid extraction step (Diasorin Liason, Abbot ID Now)
- Please check with LabCorp and Quest before sending them samples in PrimeStore MTM. If they say they will not process your samples, ask them "why not?"
BUT
- Samples can be used on POC platforms such as GeneXpert, LIAT and Biofire, as well as respiratory multiplex and syndromic panel platforms without processing under a containment hood.
To get a quote for the products or to place an order, please email Sales@2BScientific.com.