New publication on Calixar’s stabilization expertise for native MPs
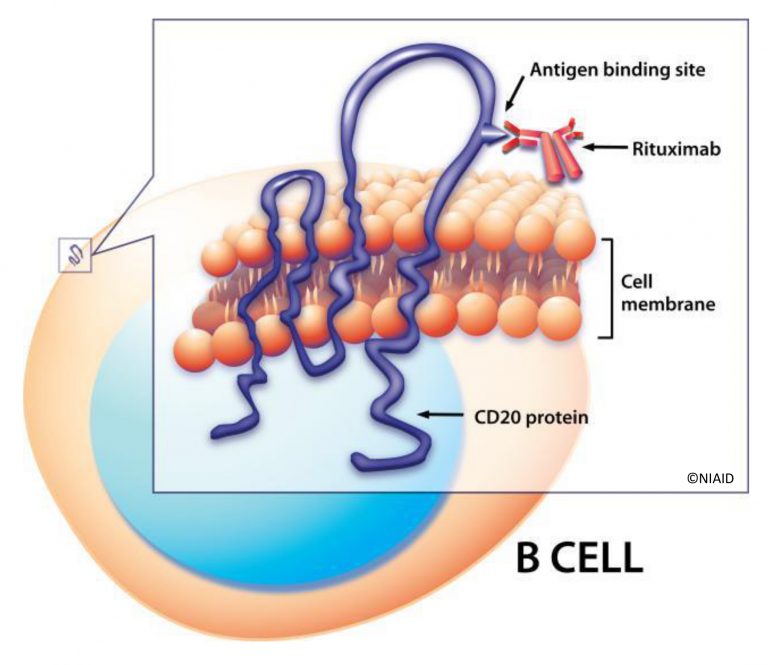
Calixar announces the publication of a new article by Calixar in collaboration with Roche Pharmaceuticals and The University of Basel (Biozentrum) in Scientific Reports on the extraction and purification of native CD20, a B-lymphocyte specific integral membrane protein (Agez M et al., 2019, Scientific Reports: “Biochemical and biophysical characterization of purified native CD20 alone and in complex with rituximab and obinutuzumab”).
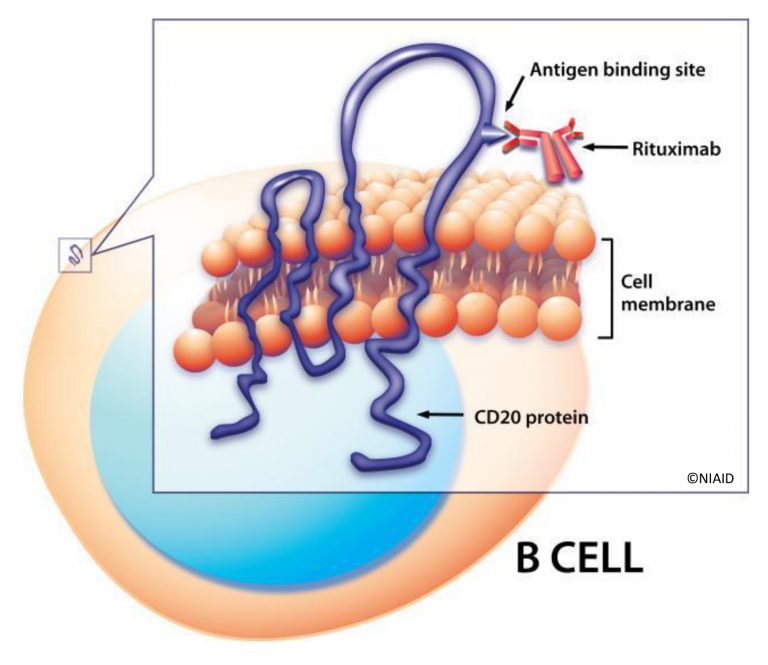
In this article, we report the extraction and purification of native CD20 from SUDHL4 and RAMOS cell lines. To improve the protein yield, we applied a calixarene-based detergent approach to solubilize, stabilize and purify native CD20 from HEK293 cells. Size Exclusion Chromatography (SEC) and Analytical Ultracentrifugation show that purified CD20 was non-aggregated and that CD20 oligomerization is concentration dependent.
Negative stain electron microscopy and atomic force microscopy revealed homogenous populations of CD20. However, no defined structure could be observed. Interestingly, micellar solubilized and purified CD20 particles adopt uniformly confined nanodroplets which do not fuse and aggregate. Finally, purified CD20 could bind to rituximab and obinutuzumab as demonstrated by SEC, and Surface Plasmon Resonance (SPR).
Specificity of binding was confirmed using CD20 antibody mutants to human B-cell lymphoma cells.
The strategy described in this work will help investigate CD20 binding with newly developed antibodies and eventually help to optimize them. This approach may also be applicable to other challenging membrane proteins.