The rise of recombinant antibodies in the reagents market
Written by Ian Wilkinson
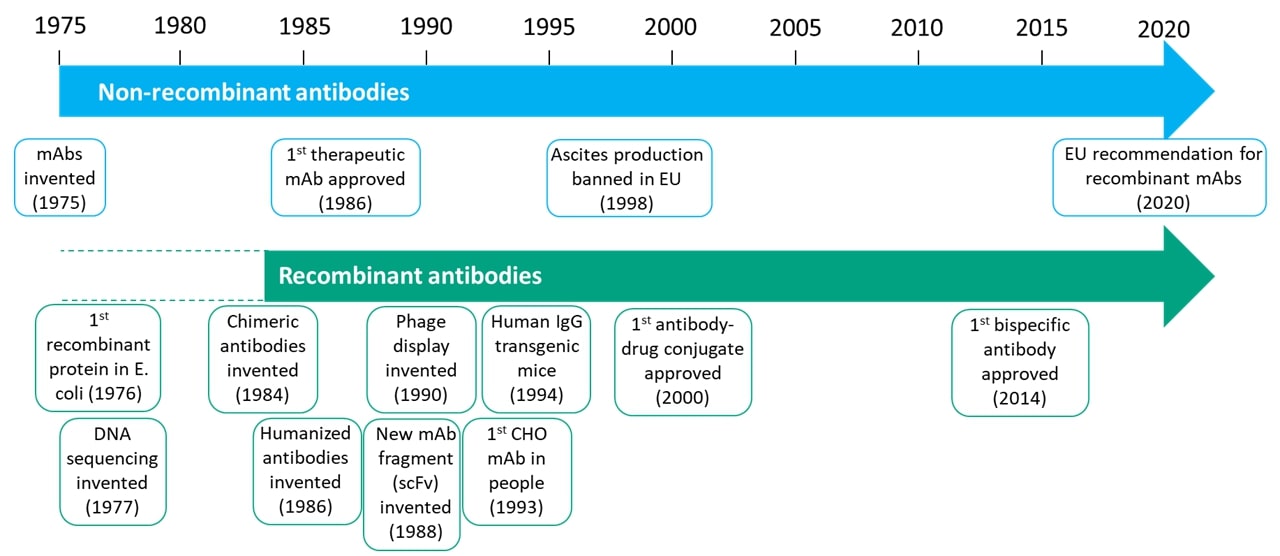
The invention of monoclonal antibodies in 1975 (1) was a pivotal moment for biological sciences and accelerated the development of markets covering research reagents, diagnostics and therapeutics that, between them, are now worth many hundreds of billions of dollars. This single invention enabled the routine discovery of highly specific and potent targeting agents of high quality that can be used in a myriad of different ways. This did not come without challenges though and in the 1980s led to a split in the discovery and manufacture of monoclonal antibodies that is still in place today.
Monoclonal antibodies were originally produced from hybridomas, which are fusions of antibody-secreting B-cells with a type of cancer cell known as a myeloma. Animals, mostly rodents in the early days of antibody development, were immunised with the target of interest and then raised an immune response to this. In very simple terms, the B-cells were harvested, fused to myelomas to create a panel of hybridomas and screened to select monoclonal antibodies of interest. The species of the resulting antibody is determined by the immunised host. For therapeutic use, this created a major dilemma. Although the first antibodies tested and even approved for use in people were of mouse origin – the anti-CD3e antibody muromonab was approved for transplant rejection in 1986 – it quickly became apparent that the use of non-human antibodies in patients can induce a host immune response targeting the non-human antibody and removing it from their system rapidly. Fortunately, at around the same time, Frederick Sanger invented DNA sequencing (2) and the fields of molecular biology, and recombinant protein production were starting to take off. Ultimately these fields merged to create recombinant antibodies and the new field of biotechnology known as antibody engineering. Hybridomas could be sequenced to identify the DNA coding sequence for the antibody and then this could be produced synthetically in cell lines such as Chinese hamster ovary (CHO) or human embryonic kidney 293 (HEK293). Knowing the sequence of the antibody opened up the possibility of manipulating it, which led to the invention of chimeric and then humanised antibodies. These contain part rodent and part human sequence, with chimeric being more human than the original rodent antibody and humanised even more so. From the mid-80s onwards, the therapeutic antibody field never looked back. All therapeutics are now manufactured recombinantly, even if the original discovery process involves the use of animals for hybridoma generation
The same wholesale switch to recombinant antibodies has not taken place in the research reagent or diagnostic markets. Unlike with therapeutics, there wasn’t a necessity for it, and so the added complexity, time and cost created a barrier that prevented the adoption of the technology. Antibody engineering experienced a renaissance from the mid-80s onwards, as clearly shown in figure 1, with almost all the progress in antibody discovery, engineering, development, manufacturing and analysis coming in the recombinant focussed therapeutics space. The reagent and IVD sectors languished behind.
The dawn of recombinants as reagents
In more recent years, a number of factors have led to the gradual emergence of recombinant antibodies in the research reagent and diagnostic sectors. The first is scientific and is part of what has been dubbed ‘The reproducibility crisis’. Although the origins of this movement are older, its rise to prominence can be pinpointed to a series of publications in 2015. These highlighted the immense amount of wasted money and time that can be attributed to low-quality reagents, mostly antibodies (3-8). One of the recommendations that came out of these publications was the shift to recombinant antibodies to ensure higher quality, reproducibility, traceability and security.
The second factor driving the reagents market towards recombinants was the increasing desire and demand to reduce the use of animals and the changing regulatory landscape. Within the EU, the manufacture of monoclonal antibodies via the ascites method, where monoclonal antibodies are grown within the peritoneal cavity of a rodent, was banned in 1998, and in 2020, the EU Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM) published a recommendation stating “animals should no longer be used for the development and production of antibodies for research, regulatory, diagnostic and therapeutic applications….EU countries should no longer authorise the development and production of antibodies through animal immunisation, where robust, legitimate scientific justification is lacking” (9).
The speed with which the reagents market is adopting recombinant antibodies is clearly accelerating, with most large suppliers now offering at least a proportion of their antibodies in recombinant form. But why should end users care, and what are the advantages of transitioning to recombinant antibodies?
What is a recombinant antibody?
Simply put, a recombinant antibody is one that is manufactured in vitro using synthetic genes and, as such, does not require the use of animals in the production process (although animal components such as fetal bovine serum may or may not be utilised). Traditional monoclonal antibodies manufactured from hybridomas are not recombinant but can be converted into a recombinant form by sequencing the hybridoma DNA/RNA encoding the antibody or alternatively direct sequencing of the antibody protein. This can create some confusion as different manufacturers and users will use the term recombinant slightly differently. To explain this requires a more detailed understanding of antibody discovery and manufacturing processes.
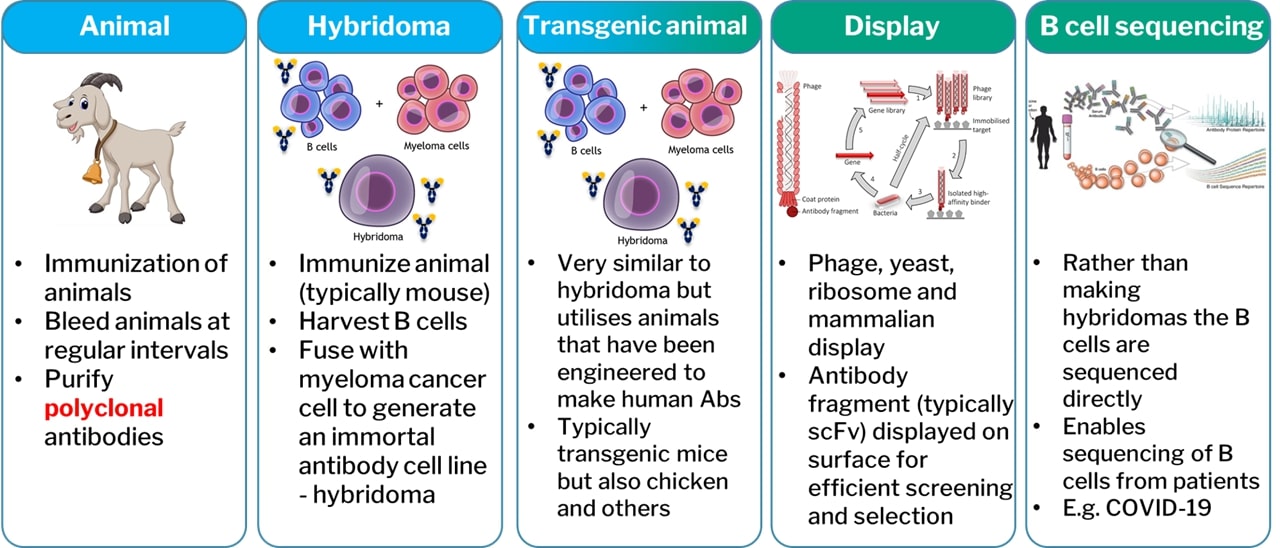
In very basic terms, antibodies can be discovered by the five techniques shown in figure 2, although the author notes that technology is developing rapidly in the discovery space with things like true de novo in silico antibody discovery on the horizon. Animals are utilised in the creation of polyclonal antibodies or monoclonals through the use of hybridomas. These are non-recombinant discovery platforms. The other platforms utilise synthetic genes and so are recombinant. This does not necessarily preclude the use of animals – in fact, very few antibody discovery processes are truly animal free. When some manufacturers refer to recombinant antibodies, they are specifically referring to those that have been discovered by a recombinant technology. However, this is not strictly correct, and whether an antibody is recombinant or not is determined by how it is manufactured and not how it was originally discovered. This means any antibody from any source can be converted into a recombinant form.
For non-recombinant antibodies, the discovery process and manufacturing platform are essentially the same thing. In other words, the immunised animal or hybridoma can be utilised for the production of the reagent. However, when dealing with recombinant antibodies, or recombinant proteins in general, alternative manufacturing processes are required that utilise a host cell to produce the protein from the synthetic genes. This could be a bacteria, yeast or insect cell, but typically, when dealing with complex proteins like antibodies, it will be a mammalian cell, most likely a HEK293 or CHO cell line.
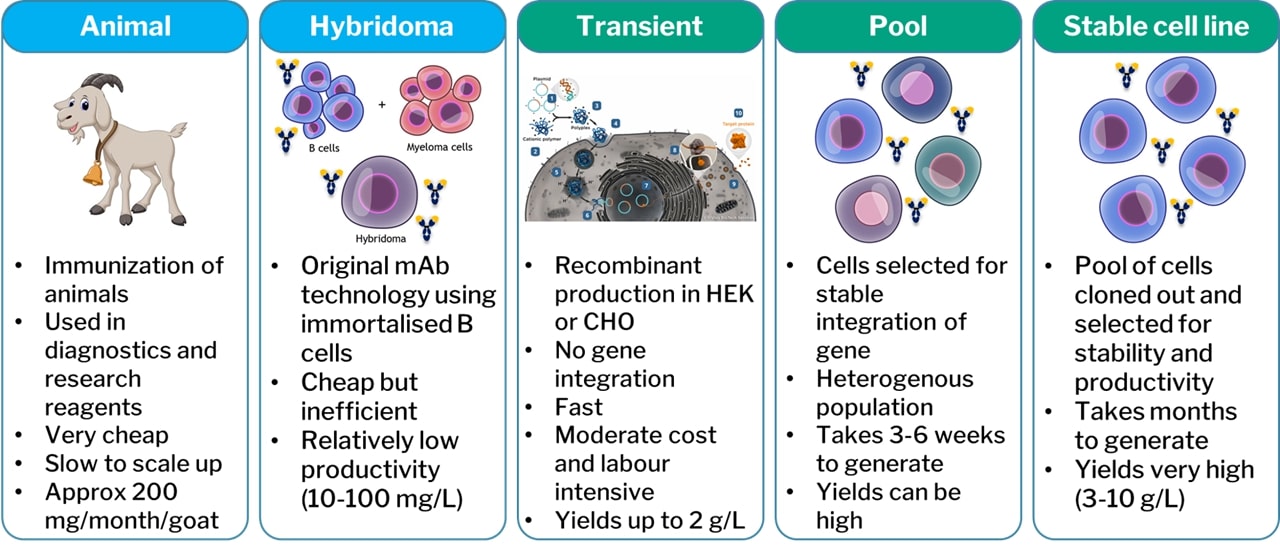
DNA plasmids encoding the antibody of interest can be introduced to such cell lines, which will then produce the antibody. This process can be broken down further into three categories (figure 3): transient expression; pools; and stable cell lines. Antibody encoding plasmids are transfected into the host cells by one of numerous methods that are outside of the scope of this paper. For a period of 1-2 weeks post-transfection the host cell will express the antibody encoded by the plasmid and secrete the antibody into the supernatant. This is transient expression. It is quick, i.e., antibody can be obtained within weeks, and relatively cost effective. The draw backs tend to be lower expression yields and lack of scalability. For this reason, transient expression is typically utilised for milligram to low gram quantities of antibody.
A very small proportion of the antibody encoding plasmid will be randomly integrated into the host genome. If the plasmid also incorporated an antibiotic resistance marker or other means of selecting these cells, then these can be isolated (typically by killing off all cells that have not integrated the plasmid). This creates a stable pool of cells, all of which have integrated the antibody plasmid randomly into their genomes. Such a pool can be generated in a matter of weeks and is a useful option for generating larger quantities of product for more prolonged periods of time. The very nature of a pool though means that all the cells are different with different copy numbers of the plasmid at different integration sites. Some cells will be more stable and higher yielding than others. Through more laborious efforts, typically taking 3-6 months, the best individual cell can be isolated to create a stable cell line. For large scale manufacture, this is ideal, but the cost and timelines can be prohibitive within pre-clinical research or the reagents market. For these reasons, transient transfection is often the preferred method of manufacture in these sectors.
Advantages of recombinant antibodies
Although hybridomas have been the default production platform within the reagents sector for decades they do suffer from a number of inherent risks and disadvantages. The antibody sequence is unknown. Hybridomas can undergo genetic drift, are easily cross-contaminated and, to the surprise of many, are often not actually monoclonal (10). Without knowing the sequence, it can be difficult or impossible to control this, leading to batch-to-batch variability, which is widely known to be an issue for end-users. Hybridomas also do not offer security over long-term supply. Cell lines can die off or stop secreting, liquid nitrogen tanks can become empty, or facilities damaged by fire and other incidents. Finally, hybridomas are of a fixed species and format. If the antibody selected is a mouse IgG1 then you are stuck with this, even if a mouse IgG2a or human IgG1 would have been preferable for your experiments.
All of these limitations and more are overcome by recombinant technology. Recombinant antibodies are defined at the sequence level and thus with the appropriate controls in place can ensure consistency across batches. With appropriate backups and especially with cloud storage a sequence provides ultimate security of an antibody and removes the need for expensive liquid nitrogen storage facilities. Of course, reproducing an antibody with consistency and long-term security offers some benefits to end-users, but this is really just the beginning. The real power of recombinants is the possibility to explore opportunities that are simply impossible with traditional hybridoma antibodies. As the sequence is known, it can be reproduced identically to the original antibody, or alternatively it can be engineered for improved performance. Antibody species, isotype or subtype can be readily swapped to better suit experimental needs. Antibodies can be engineered into alternative design formats such as fragments, bispecifics or fusion proteins. Fc effector function and half-life can be modulated. Antibodies can be humanised and/or affinity optimised. No longer is it the simple rule of one hybridoma equals one antibody. That one parental antibody can be relatively quickly and easily modified into a multitude of different options that are tailor-made for specific applications and uses. All of this combined potentially enables more sensitive, reliable and accurate experiments.
Conclusion
The wholesale switch to recombinants that took place in the therapeutics arena in the 1980s has not yet taken place in the reagents sector, but the journey towards that did start 10-15 years ago and now appears to be picking up pace. Where once it was unusual to find a supplier that provided recombinant antibody reagents, it is now unusual to find one that does not. Although, for many, it is still only a small proportion of their reagents that are recombinant, this is a clear signal of the direction of travel of the market. This should lead to better quality reagents and open up greater possibilities for scientists to tailor their antibody reagents to their own specific needs.