Human Cytokine Products
1. Highly Active Human Interferon Protein
High quality recombinant proteins derived from bacterial or mammalian cells
- Consistent performance and biological activity
- 95% or greater purity
- Endotoxin level < 1 EU/μg
- Suitable for use in a range of applications including ELISAs & cytopathic effect assays
Key Benefits of PBLs IFN Elisa Kits are:
- Best in class kits that support different research needs
- Extensively used as they are highly sensitive and can detect the lowest levels if IFN subunits
- Validated by many CROs and has been development by testing with diseased samples
- Can detect the lowest level of LLOQ all disease sample matrix therefore robust matrix tolerance
Featured:
Universal Type I Interferon (Cat. No. 11200)
Unique hybrid interferon exhibiting activity across multiple species
- Useful for cross-species comparative studies of Type I IFN effects
- Broad crossover to multiple animal models in vivo and in vitro where the species relevant Type I IFN-α is unavailable or performs inconsistently
- High specific activity on multiple animal cell lines
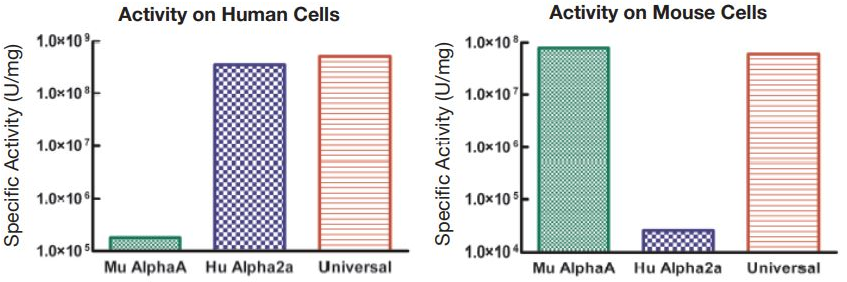 Figure 1. Comparison of the Activities of Universal Type I IFN, Human IFN Alpha 2a, and Mouse IFN Alpha A
Figure 1. Comparison of the Activities of Universal Type I IFN, Human IFN Alpha 2a, and Mouse IFN Alpha A
2. Human Interferon Alpha (IFN-α) ELISAs
Featured:
Human IFN Alpha All Subtype ELISA (Cat. No. 41115)
High Sensitivity – can detect lowest level of subunits
- Assay Standard Range: 1.95 – 125 pg/ml
- Accurate low pg/ml detection of all human IFN-α subtypes
- Robust matrix tolerance including autoimmune disease sera, normal serum, and tissue culture media (TCM)
- Optimized for autoimmune serum and plasma samples
- Consistent, reproducible results with < 10% inter- and < 8% intra-assay CVs
- Detects previously “missed” IFN-α subtypes
Table 1. Lower Limit of Quantitation (LLOQ) and Lower Limit of Detection (LLOD) in Normal Human Serum for all 12 IFN Alpha subunits including a Absorbance Chart at 450nm
| Subtype | LLOQ (pg/ml) | LLOD (pg/ml) |
|---|---|---|
α1 (αD) | 2.31 | 0.76 |
α2a (αA) | 1.36 | 0.42 |
α4a (αM1) | 1.38 | 0.43 |
α5 (αG) | 1.70 | 0.51 |
α6 (αK) | 1.13 | 0.33 |
α7 (αJ1) | 2.09 | 0.59 |
α8 (αB2) | 2.81 | 0.82 |
α10 (αC) | 1.77 | 0.65 |
α14 (αH) | 1.76 | 0.51 |
α16 (αWA) | 2.01 | 0.68 |
α17 (αI) | 1.36 | 0.50 |
α21 (αF) | 6.18 | 1.67 |
● Good ● ● Better ● ● ● Best | ||||
Global IFN-α subtype measurement in serum or plasma | ● ● | ● ● ● | ||
Global IFN-α subtype measurement in TCM | ● ● | ● ● | ● ● ● | |
Reproducible results with < 10% inter- & intra-assay CVs | ● ● ● | ● ● ● | ● ● ● | ● ● ● |
3.Human Interferon Beta (IFN-β) ELISAs
Featured:
Human IFN Beta Serum ELISA (Cat. No. 41415)
High Sensitivity
- Assay Standard Range: 1.2 – 150 pg/ml
- Provides > 90% serum spike-recovery
- Reproducible results with < 8% inter- and intra-assay CVs
- Robust matrix tolerance for autoimmune disease sera
- Validated by multiple CROs for sensitive measurement of IFN-β therapeutics in human serum samples
- e.g. Rebif®, Avonex®, Extavia®, Betaseron®
- Suitable for PK, PD, Toxicity & Biomarker studies
Related Products
Cat. No. | Description | Size |
To learn more, please contact Info@2BScientific.com or call us at +44 (0)1869 238033!
● Good ● ● Better ● ● ● Best | ||
Accurate IFN-β measurement in serum or plasma (including autoimmune disease sera) | ● ● ● | |
Accurate IFN-β measurement in tissue culture media (TCM) | ● ● | ● ● ● |
Reproducible results with < 10% inter- & intra-assay CVs | ● ● | ● ● ● |
4. Anti-Human IFN Monoclonal (MAb) & Polyclonal (PAb) Antibodies
Labeled & unlabeled antibodies for a variety of uses
- MAbs offer high specificity in applications where specific binding is desirable
- PAbs are preferable for neutralization because they target a variety of specific epitopes of a particular antigen
Featured: Anti-Human IFNAR2, Clone MMHAR-2 (MAb) (Cat. No. 21385-1)
- Blocks biological activity of human Type I IFNs and IFN-α receptor.
- Binds to human IFN-α receptor with high affinity.
Figure 5. Mouse MAb to Human IFN-Alpha/Beta Receptor 2.
Human Type I IFN Neutralizing Antibody Mixture (Cat. No. 39000)
Mixture of MAbs and PAbs directed against human Type I IFNs & IFNAR2
- Effectively neutralizes biological activity of human IFN-α, IFN-β, IFN-ω, IFN-κ, and IFN-ε.
- Improves neutralization of multiple Type I IFNs often found in complex samples by employing a broadly neutralizing custom antibody cocktail.
Figure 6. Representative neutralization curves (diluted 1:50) to human IFN-α, -β, and -ω. A549 cells incbated with antibody mixture and titrated IFN, subsequently challenged with EMCV.