StressMarq Biosciences
Established in 2007, StressMarq Biosciences Inc. is a supplier of life science products that operates out of Victoria, Canada with a small, but dedicated group of scientists. Headed by the CEO and President Dr. Ariel Louwrier, StressMarq provides the research community with high-quality reagents backed with rigorous quality control data, expert scientific support, and fast international delivery.
View All StressMarq Biosciences Products
StressMarq is the market leader in the development and commercialization of a range of unique fibrilized and oligomeric protein preparations for use in neurodegenerative disease research, including proteins such as alpha synuclein, tau, amyloid beta, SOD1 and TTR. StressMarq’s strategic efforts are focused on expanding the breadth and depth of this product offering to assist researchers studying Alzheimer’s, Parkinson’s, and other neurodegenerative diseases.
In the years to come, StressMarq will continue to aid life science research by providing “Discovery through partnership, and Excellence through quality”.
In addition to our focus on products for neurodegenerative disease research, our diverse portfolio of antibodies, proteins, kits and small molecules are utilized across a range of life sciences including neuroscience, cancer research and cell signaling.
To aid research worldwide, StressMarq products are available through an extensive network of international distributors in over 45 countries.
StressMarq Custom Forms
Stressmarq Featured Products
New Active Synuclein Protein Fibrils and Monomers
We have developed active alpha synuclein protein fibrils that can be used to induce endogenous alpha synuclein phosphorylation and subsequent Lewy body inclusion formation in neuronal cell culture or for in vitro oligomerization studies.
Our active alpha synuclein protein fibrils seed the formation of new fibrils from active alpha synuclein monomers.
Type 1 human and mouse preformed fibrils (PFFs) and monomers have been tested in cell culture assays and thioflavin T assays. Human and mouse PFFs have been tested in vivo and produced alpha synuclein pathology in rat brains within 30 days of injection. A53T alpha synuclein monomers and PFFs are also available. The A53T mutation has been linked to early-onset Parkinson’s Disease and increased alpha synuclein fibrillization.

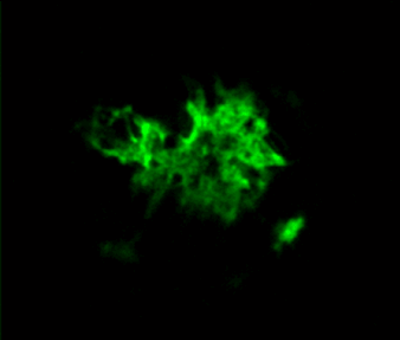
Rat primary hippocampal neurons treated with Type 1 mouse alpha synuclein PFFs showing Lewy body inclusions.
 IHC analysis of rat brain injected with Type 1 mouse alpha synuclein PFFs (SPR-324) shows alpha synuclein pathology.
IHC analysis of rat brain injected with Type 1 mouse alpha synuclein PFFs (SPR-324) shows alpha synuclein pathology.
Alpha synuclein oligomers are increasingly thought to be the toxic species in synucleinopathies. Dopamine and EGCG can be used to stabilize alpha synuclein in its oligomeric forms, preventing further aggregation into fibrils.
StressMarq's Active Tau Pre-Formed Fibrils (PFFs)
StressMarq is excited to offer active tau proteins to help researchers study tau aggregation, a hallmark of neurodegenerative diseases including Alzheimer’s. StressMarq is the first to offer active tau preformed fibrils (PFFs) and filaments for neuroscience research. The process of tau aggregation can be seeded by active tau PFFs, which recruit monomers to form larger tau fibrils. This has been demonstrated in thioflavin T assays where an increase in fluorescence, indicative of tau fibrillization, is seen when active tau PFFs are combined with active tau monomers. Certain tau PFFs have been injected into P301L mice, where they seed tau aggregation and induce tau pathology in the hippocampus.
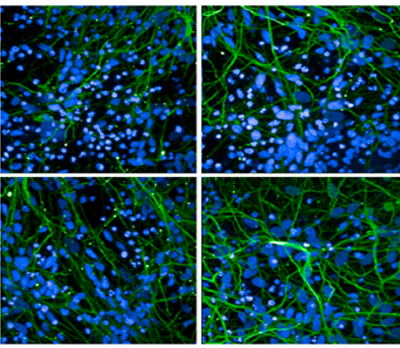
Immunohistochemistry analysis of P301L mouse hippocampus injected with K18 P301L tau PFFs (SPR-330) shows seeding of tau pathology at injection site nine weeks post-injection. AT8 (pSer202/pThr205) tau antibody shows tangle-like inclusions. Inset: negative control. Experiments performed at reMYD N.V.
Monomers and fibrils are available both in the full-length isoform of the tau protein (2N4R or Tau-441) or a truncated form (K18). Tau-441 has a molecular weight of approximately 46 kDa, whereas K18 tau has a molecular weight of approximately 15 kDa. dGAE is a fragment of the tau protein consisting of amino acids 297-391. It is one of the core PHF subunits,1 includes both microtubule-binding domains and proline-rich regions, and assembles into PHF-like fibrils in vitro without additives or templates.
Proteins are available as wild-type or with a variety of mutations. P301S and P301L mutations occur in exon 10 and are associated with frontotemporal dementia. The P301S mutation reduces tau’s ability to assemble microtubules, and the P301L mutation promotes beta-sheet formation and the formation of PHFs. Both P301S and P301L mutant transgenic mouse models are used in tau research. The K280 deletion mutation is also associated with frontotemporal dementia and promotes fibrillization into paired helical filaments (PHFs) in the absence of heparin and other inducers. The C322A mutation also increases tau’s ability to form PHFs.2
PFFs induce tau aggregation; full-length PFFs may be more effective in seeding fibrillization, but a combination of both can be particularly toxic to neurons. Most tau varieties are fibrillized using a heparin scaffold; soluble tau filaments are fibrillized using a linear anionic scaffold. dGAE tau and K18 K280 deletion tau both fibrillize without scaffolds.
Tau PFFs can be expressed in baculovirus in addition to E. coli. Baculovirus tau PFFs are post-translationally modified, fibrillize without a scaffold, and are endotoxin-free*.

-in-SK-N-BE-cells.png?width=50)